As previously discussed, the concept of Quality by Design is a proactive process to identify the errors that matter when doing a randomised trial. This is in contrast to ICH-GCP which focuses considerable time and effort through data checking to ensuring all data are error free. ICH-GCP regards all errors as important, whereas QbD sets out to identify which errors matter when doing a trial and which are not important.
The Heart Protection Study: Data verification – focusing on things that don’t really matter
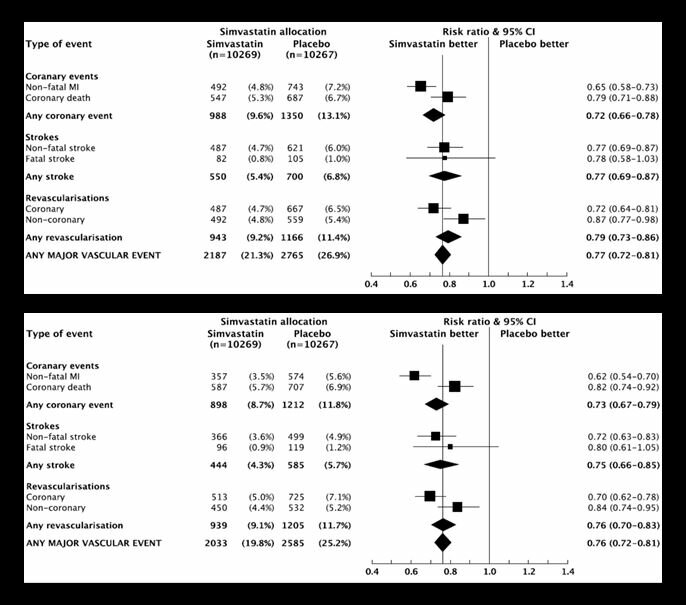
The MRC/BHF Heart Protection Study was a landmark randomised trial completed in the 1990s that showed the benefits of lowering-cholesterol in over 20,000 individuals at high-risk of vascular disease. The results above show the benefit of cholesterol-lowering with simvastatin for a wide-range of vascular outcomes.
ICH-GCP is not an ethical standard for research
ICH-GCP states that it is an ethical standard for the design and conduct of research, but close inspection of the guideline leads to the conclusion that it is concerned with the process of ethics, specifically, the process of ethical review rather than the core principles of ethics. This is a pity because the principles that underpin research ethics are essentially straightforward and easy to grasp. I will try to outline them here.
Fundamental problems with ICH-GCP: #5 – part 2 – Hiding behind complexity
I started writing this piece a few weeks back on holiday in the Algarve. It’s a follow on to the last piece on mindlessly following a recipe rather than thinking what matters when doing a trial. This time it looks at the value of simplicity.
I went for a quick run this morning before it got too hot. When I got back to our villa I jumped straight in the shower. Ten minutes later I was sitting down with a coffee chatting with my wife about what we might do today.
What the hell does this have to do with randomised trials I guess you might well be thinking?
Fundamental problems with ICH-GCP: #5 – part 1 – It’s not painting by numbers
This is a personal perspective on what I think is one of the fundamental problems with the whole paradigm of how we regulate randomised trials. This is the mindless following of instructions rather than thinking about what matters to do a trial well.